


Group Meeting









|
Current
and Future Activities of Our Research Unit
|
 |
| PREPARATION AND CHARACTERIZATION OF SILK FIBROIN NANOPARTICLES |
Silk fibroin fiber is a natural protein with various special properties. In this research, silk fibroin
nanoparticles were prepared by precipitating silk fibroin solution in organic solvents. The molecular
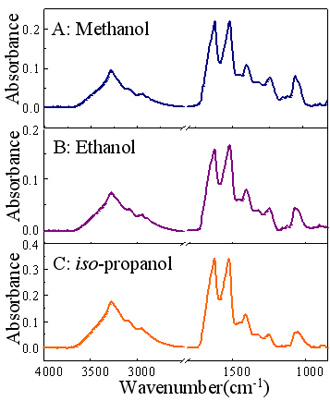
characteristics of silkfibroin nanoparticles were investigatedusing ATR FT-IR microspectroscopy.
It was found that silk fibroin nanoparticlestreated with methanol had the highest crystalline content
|
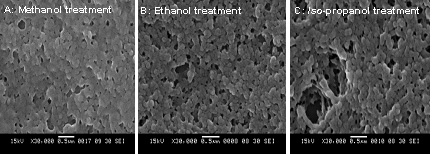
SEM micrographs of silk fibroin nanoparticles obtained by precipitation method :
(A) Silk fibroin nanoparticles treated with methanol,
(B) Silk fibroin nanoparticles treated with ehanol, and
(C) Silk fibroin nanoparticles treated with iso-propanol.
|
References:
1. Joo Y., European Polymer Journal, 39, 1195-1199 (2003).
2. Emilia B., Spectrochimica Acta Part A, 62, 105-111 (2005).
3. Xiao H., Macromolecule, 39, 6161-6170 (2006).
|
PREPARATION AND CHARACTERIZATION OF SILK SERICIN MICROPARTICLES |
Silk sericin microparticles were prepared by precipitation
in organicsolvent (methanol, ethanol and iso-propanol).
Effect of organic solvent on the molecular characteristics
of silk sericin microparticle were investigatedby ATR FT-IR
microspectroscopy.The results suggested that the conformation
of the microparticle were changing from random coil to b-sheet
and b-turn afterthe treatment. After being treated with methanol,
ethanol and iso-propanol,the b-sheet and b-turn proportion of
microparticles were increased.The appearance of b-sheet and
b-turn conformation may be associated with the packing of
molecular chains.
References:
1. Y.Q. Zhang, Biotech Adv., 2002, 20, 91-100.
2. J.H. Wu, Z. Wang and S.Y. Xu, Food Chem.,
2007, 103, 1255-1262.
3. X. Hu, D.Kaplan and P. Cebe, Macromolecules,
2006, 39, 6161-6170.
4. K.Y. Cho, J.Y. Moon, Y.W. Lee, K.G. Lee,
J.H. Yeo, H.Y. Kweon, K.H. Kim and C.S.
Cho, Biol Macromol., 2003, 32, 36-42.
5. K. Lee, H.Y. Kweon, J.H. Yeo, S.O. Woo,
Y
.W. Lee, C.S. Cho, K.H. Kim and Y.H. Park,
Biol Macromol., 2003, 33, 75-80.
6. A. Kurioka, F. Kurioka and M. Yamazaki,
Biosci Biotech Biochem., 2004, 68, 774-780.
|
|
SECONDARY STRUCTURE OF SINGLE SILK FIBER OBSERVED BY ATR FT-IR MICROSPECTROCSOPY |
Silk is a natural fiber with high strength and good mechanical properties.
The properties of silk fiber depend on the quality of silk cocoon. The structure of
silk in each layer is well be obtained when the secondary structure of silk can be
investigated. Moreover, the study of single fiber of silk cocoon by ATR FT-IR
microspectroscopy is necessary for constructing the data base of Thai silk.
ATR FT-IR technique is non-destructive, rapid analysis and sample preparation
is not required
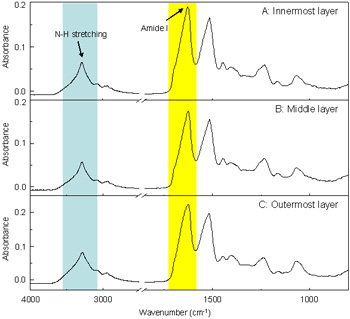
ATR FT-IR spectra of silk cocoon fiber at innermost (A), middle (B) and outer layer (C).
References:
1. C. Holland., A.E. Terry. and D. Porter., F. Vollrath. Polymer, 1-5 (2007).
2. G. Freddi., R. Mossotti. and R. nnocenti. Journal of Biotechnology, 106, 101-112 (2003).
3. M. Kevin. and K. David. Protein-Based Materials, Birkhäuser Boster, 1997.
4. Z. Hong-Ping., F. Xi-Qiao., C. Wei-Zheng. and Z. Feng-Zhu. Engineering Fracture Mechanics, 2006.
|
PLASMONICS OF SPHERICAL SILVER NANOPARTICLES SIMULATED BY MIE THEORY |
Theoretical simulation based on Mie Theory of spherical silver nanoparticles
in water gave many insights to the optical properties of the particle. The underlying principles
of these properties are the interactions between conduction electrons on nanoparticles,
in terms of collective oscillation, and incident electromagnetic radiation. When the particle
size is changed, the electron distribution around the surface is also changed. Therefore,different
optical properties of the nanoparticles are exhibited. The surrounding medium at the vicinity of
nanoparticle
surfaces are also contributed the variation to the electron distribution. So, depend-
-ing on material (i.e. the refractive
index of the material), the nanoparticles will response to
this change differently. For example, in the case of air coated nanoparticles, when the thickness
of air layer is increasing, the dipole plasmon resonance is observed at the shorter wavelength, and
the maximum extinction efficiency is decreased (i.e. compared to the bare particles)
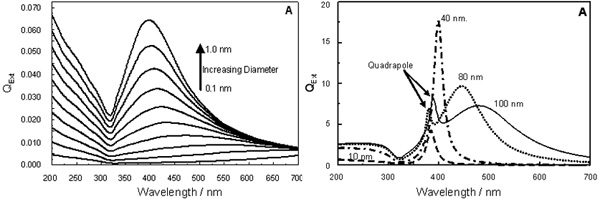
(Left) : Extinction efficiencies for silver nanoparticles of size 0.1 nm to 1.0 nm, step of 0.1 nm, in water.
(Right) : Extinction efficiencies10, 40, 80 and 100 nm
References:
1. P. B. Johnson and R. W. Christy, Physical Review B, 1972, 6, 4370 - 4379
2. A. D. Rakic, A. B. Djurisic, J. M. Elazar and M. L. Majewski, Applied Optics,
1998, 37, 5271 -5283.
3. G.F. Bohren and D. R. Huffman, "Absorption and Scattering of Light by Small Particles",
John Wiley & Sons, Inc., 1983.
4. C. Noguez, Optical Materials, 2005, 27, 1204 - 1211.
|
SYNTHESIS OF HIGH CONCENTRATION COLLOIDAL SILVER NANOPARTICLES |
This research has a major aim at developing a technique for producing
large amount of controllable size, highly concentrated (10,000-100,000 ppm) and highly
stabilized silver nanoparticle. A nebulization or atomization technique was employed for
the nebulization of solution of silver salt into the solution of a reducing agent. The plasmo-
-
nabsorptions of high concentration of silver nanoparticles were measured. The plasmon
absorptions indicated that the synthesized silver nanoparticles has smaller size and narrow
size distribution then these of the commercial due to the lower plasmon band
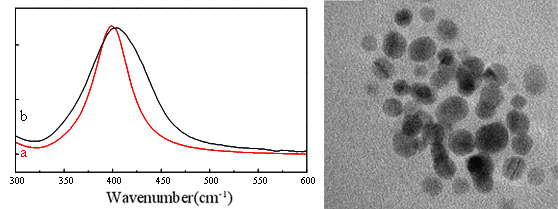
(Left) : The plasmon absorption bands of diluted 1,000 to 10,000 from high
concentration of silver colloid
(a) Our synthesized silvernanoparticles (lMax = 399 nm).
(b) Commercial product (Max = 405 nm).
(Right) : TEM image of synthesized silver colloid has mean diameter of ~10 nm.
References:
1. D.L. Van Hyning and Zukoski C.F "Formation mechanisms and aggregation behavior of
borohydride reduced silver particles." Langmuir, 1998, 14, pp. 7304-7046.
2. W. Hongshui, Q. Xueliang, C. Jianguo, W. Xiaojian and D. Shiyuan "Mechanisms of PVP
in the preparation of silver nanoparticles" Mater. Chem. Phys. 2005, 94, pp. 449-453.
3. P. Raveendran, J. Fu and S.L. Wallen "Completely "Green" synthesis and stabilization of
metal nanoparticles" J. Am. Chem. Soc. 2003, 125, pp. 13940-13941.
4. B.J. Wiley, S.H. Im, Z.-Y. Li, J. McLellan, A. Siekkinen and Y. Xia "Maneuvering the surface
plasmon resonance of silver nanostructures through shape-controlled synthesis" J. Phys. Chem. B
2006, 110, pp. 15666-15975.
|
PADDING OF SILVER NANOPARTICLES ON COTTON AND POLYESTER
AND THEIR ANTIBACTERIAL EFFICACY |
Silver nanoparticles are non-toxic and antibacterial agent. In this work, the antibacterial efficacy
of silver nanopartilces on cotton and polyester was investigated. Padding process has been used for
the immobilization of silver nanoparticles onto the surfaces cotton and polyester fabric. The size, size
distribution and morphology of nanoparticles was characterized by UV-visible spectroscopy and
transmission electron microscopy (TEM). The silver sol had narrow size distribution with the average
size of 5-10 nm. At low padding concentrations (20-50ppm), silver nanoparticles show excellence
antibacterial activity.
References:
1. D. P. Dowling, K. Donnelly, M. L. McConnell, R. Eloy, and M. N. Arnaud, Thin Solid Films,
2001, 398-399, 602-606.
2. J. M. Schierholz, L. J. Lucast, A. Rump, G. Pulverer, J. Hosp. Infect. 1998, 40, 257-262.
3. C. Chug, M. Lee, E. K. Choe, Carbohydr. Polym. 2004, 58, 417-420.
4. S. H. Jeong, Y. H. Hwang, S. C. Yi, J. Mater. Sci. 2005, 40, 5413-5418.
5. H. J. Lee, S. Y. Yeo, S. H. Jeong, J. Mater. Sci. 2003, 38, 2199-2204.
|
APPLICATION OF ATR FT-IR MICROSPECTROSCOPY IN FOOD PACKAGING ANALYSIS |
Food packaging was analysed by the novel slide-on diamond ?IRE and slide-on Ge ?IRE combined
with the "contact and collect" technique. Contamination on a surface can be deposited onto the tip of both
mIREs by directly deposition or by using an organic liquid (i.e., mineral oil and fluorolube) to pick-up the
contaminants from the surface. This technique is non-destructive, ease to operate, and does not require
an additional sample preparation. The result is accurate and reliable as the trace contaminants on a surface
can be separated from the substrate, and characterized under the ATR mode without any interference from
the substrate. From polypropylene bag, polyethylene bag and disposable chopsticks analysis, we found that
there was oleamide on the surface of polypropylene bag. On the surface of chopsticks, there are talc and
wax that can be assigned to octadecanoic or stearic acid. However, we did not find a contamination on
polyethylene bag.
References:
1. S. Ekgasit, N. Pattayakorn, D. Tongsakul, C. Thammacharoen, T. Kongyou; Anal. Sci., 23 (7), 863, 2007
|
CHARACTERIZATION OF BALLPOINT PEN INKS BY ATR FT-IR MICROSPECTROSCOPY |
The dome-shaped Ge mIRE is the accessory for ATR FT-IR spectral acquisition using infrared
microscopy for forensic analysis of paper and pen inks. The dome-shaped Ge mIRE was employed.
Since contact area of the Ge mIRE is small (50x50 mm2), a trace of inks on paper can be analyzed
without an additional sample preparation. The observed spectra show unique spectral feature of each
type, brand, and color of ink. The unique spectral feature associated with chemical composition of
an individual ink can be employed for forensic applications in order to differentiate cross line of ink trace.
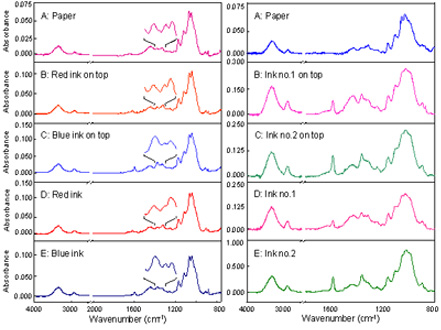
(Left)
: ATR spectra of line-crossing with different pen ink colours
(Right) : ATR spectra of line crossing with black pen ink colour
References:
1.David F.R., Chem. Soc. Rev. 2005, 34, 1021-1030.
2.Williams D.M, Forensic Sci. Int. 2005, 152, 241-247.
3.Gemma P., Talanta 2005, 67, 334-344.
4.Ashwini K., Vib. Spectrosc., 2006, 40, 270-277. |
| |
|
|
Copyright
© 2005 Sensor
Research Unit at Department of Chemistry, Chulalongkorn University
Phayathai
Road, Patumwan, Bangkok, Thailand 10330 Tel: +66-2218-7585 Updated
24/08/07
e-mail: sru@chula.ac.th
|








